02 Jan Troponin mutations
L48Q and I61Q
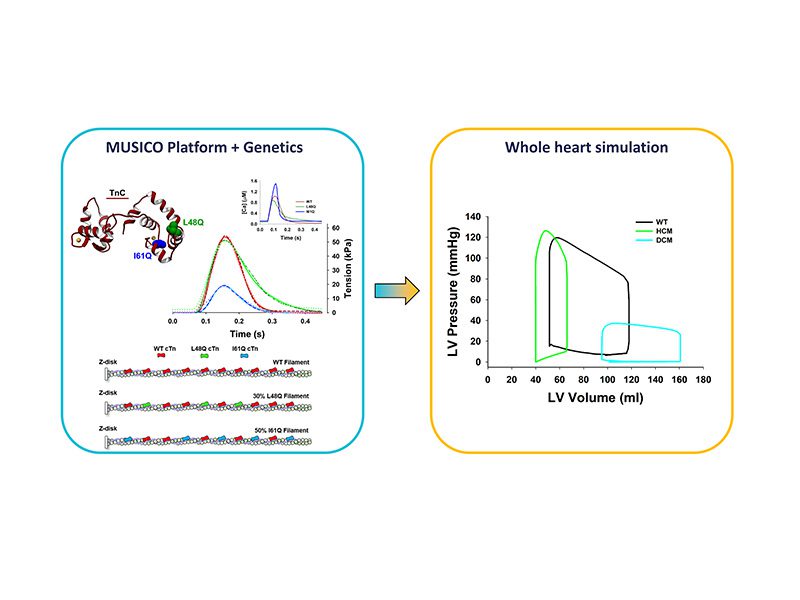
A challenge in experimental studies of muscle diseases is accounting for the effect of variable mutation incorporation into myofilaments. In this study, we used a spatially explicit computational model MUSICO Fiber, informed by experimental data from transgenic mice expressing one of two mutations in cardiac Troponin C, L48Q, related to HCM, and I61Q, related to DCM. These mutations increase or decrease calcium sensitivity which causes changes in calcium binding and dissociation from cardiac Troponin C. This is reflected in changes of intracellular calcium transients and cause prolonged tension relaxation in L48Q causing hyper contractility in HCM and much lower tension in I61Q causing hypo contraction.
In our MUSICO simulations we implemented randomly distributed mutated troponins along thin filaments with 30% of mutants in L48Q and 50% in I61Q. We demonstrated that the model can accurately describe twitch contractions for the data and explored the effect of variable mutant incorporation and localization on simulated cardiac muscle twitches.
You can read about this in more detail in our paper: Mijailovich et al. (2021), Journal of molecular and cellular cardiology, 155, 112-124.
Finally, by translating simulated data from mice to human cardiac muscles we were able to predict how these mutations affect the healthy human left ventricular Pressure-Volume loop (P-V loop).
The predicted P-V loops in HCM show lower volumes and higher pressures than normal, with significantly reduced ejection fraction. The most apparent change predicted for HCM are reduced ejection volume and slightly higher ventricular pressure comparing to healthy heart. On the other hand, the simulation for DCM hearts predicted much lower ventricular pressures caused by increased size of the heart chambers, thinner ventricle walls and reduced contractility in DCM cardiac muscle even though the peak of calcium transient was elevated. Due to increased ventricle size the P-V loops of DCM hearts are shifted toward lager ventricular volumes. Taken together, we have shown how changes at molecular scale in contractile proteins associated with HCM and DCM and the observed calcium transients can be quantified by MUSICO simulations and translated via MP surrogate model for use in FE simulations of whole heart. We have also demonstrated, though in simplified model, how these changes at molecular scale, along with observed differences in heart size and ventricular wall thickness can be translated to the functional changes observed in cardiomyopathies.




Sorry, the comment form is closed at this time.